
Services
Quality control
1
H NMR is a suitable method for detecting impurities such as solvent residues and it is widely used in the pharmaceutical, medical, and food industries for quality control.
Our laboratory is equipped for measuring liquid and solid samples, and is capable of providing measurements of a wide range of elements. Within the service, we offer measurements of elements 1H, 13C, 15N, 31P, 19F and others with higher resonance frequencies than 15N.
Compound identification
The NMR spectrum reflects connection between atoms in the molecule which makes the method ideally suited for identification of compounds. Even subtle differences in molecular configuration can be distinguished relatively easily. The method can be applied to both synthetic and natural compounds.
NMR structural analysis in liquids
Based on quantification of spin-spin couplings and nuclear Overhauser effect intensities, a complete 3D structure of compounds can be solved. However, the procedure may be laborious. Ask for details if you are interested.
Biomolecular structure and dynamics by NMR
 Proteins of up to 200 amino acids, nucleic acids up to 50 nucleotides can be studied. The necessary concentrations are at least 0.1 mM for simple tests, 0.5 mM for more complex studies. Much depends on the molecular weight, folding etc. For solving 3D structures, isotope labeling with 15N and 13C is always needed for proteins and for oligonucleotides larger that approximately 30 nucleotides.
Proteins of up to 200 amino acids, nucleic acids up to 50 nucleotides can be studied. The necessary concentrations are at least 0.1 mM for simple tests, 0.5 mM for more complex studies. Much depends on the molecular weight, folding etc. For solving 3D structures, isotope labeling with 15N and 13C is always needed for proteins and for oligonucleotides larger that approximately 30 nucleotides.
Specialized methods, some of them developed in our laboratory, for charactering inherently disordered proteins (IDPs) are available. We can measure spectra with up to 5 dimensions to assign the protein backbone and sidechain resonances.
NMR analysis in solid state
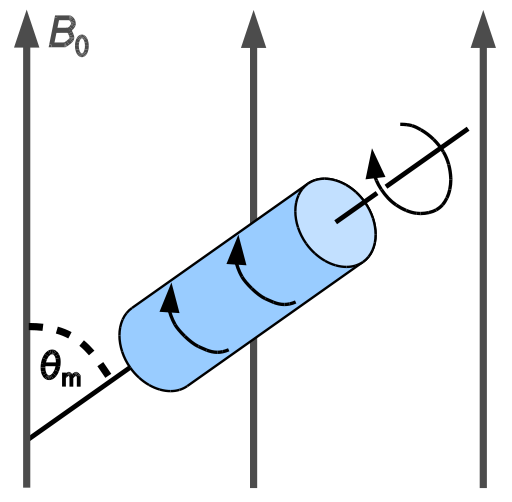 The application of solid-state NMR techniques usually arises due to specific interest in the physics of solid state, including packing effects and polymorphic structures. Solid-state NMR can be also used to study chemical shielding anisotropy. Sometimes, the application is motivated by an inability to dissolve the material of interest.
The application of solid-state NMR techniques usually arises due to specific interest in the physics of solid state, including packing effects and polymorphic structures. Solid-state NMR can be also used to study chemical shielding anisotropy. Sometimes, the application is motivated by an inability to dissolve the material of interest.
Measuring methods available and examples of their applications
 1H NMR (Proton nuclear magnetic resonance)
1H NMR (Proton nuclear magnetic resonance)
13C NMR (Carbon nuclear magnetic resonance)
13C NMR structure characterization of polyolefines
13C NMR APT (Attached Proton Test)
13 C DEPT (Distortionless Enhancement by Polarization Transfer)
COSY (COrrelation Spectroscopy)
TOCSY (TOtal Correlation Spectroscopy)
2D NOESY (Nuclear Overhauser Effect Spectroscopy)
2D ROESY (Rotating frame nuclear Overhauser Effect Spectroscopy)
2D HSQC (Heteronuclear Single-Quantum Correlation spectroscopy)
2D HMQC (Heteronuclear Multiple-Quantum Correlation)
2D HMBC (Heteronuclear Multiple-Bond Correlation spectroscopy)
Sample requirements (organic chemistry)
Solubility in one of the solvents suitable for NMR spectroscopy, preferably D2O, CDCl3, DMSO-d6, benzene-d6, methanol-d4, acetone-d6, N,N-dimethyl formamide-d7 and Dichloromethane-d7.
Approximate minimal concentrations of samples are 0.1 mM for 1H spectra and 10 mM for 13C spectra (corresponding 0.015 mg and 1.5 mg respectively, based on molecular weight 300 and sample volume 0.5 ml).
Tasks NOT suitable for NMR
Low sample quantities – the sensitivity of NMR is inherently low. Therefore, the method is NOT suitable for analysis if only very low quantity of sample is available. See Sample requirements above for minimal required concentrations.
We are not equipped for tasks requiring advanced statistical evaluation of large series of spectra such as metabolomics.
No ‘low gamma’ probes available – we have no equipment for measuring nuclei with resonance frequencies lower than 15N (50.66 MHz at 11.75 T field – 500 MHz spectrometer).



